Degree of Dissociation Formula
This compound is a _____ alkyne. A pH of more than 7 is classified as basic.
If The Degree Of Dissociation Is Given Then How To Take The Values At Equilibrium Q 2hi H2 I2 The Degree Of Dissociation Is A Calculate Expression For Equilibrium Constant Kc Of The
Three reasons for that.

. To calculate unsaturation 1 degree of saturation equals _____ hydrogen atoms which means a ring or a double bond each subtract _____ hydrogen atoms from the max. When using a strong base to titrate an acid solution of a known concentration the acid concentration can be calculated considering the fact that the neutralization reaction is complete. 1 psi 6894810-2 bar.
This is derived from the molarity of protons hydrogen ions or H in the solution. Learn about the definition and structures of crystalline structures and the. According to the degree of dissociation acids can be divided into strong acids and weak acids to form H ions when they are dissolved in water.
A pH of 7 is regarded as neutral. The pH scale which normally spans from 0 to 14 in water is used to determine the pH of an aqueous solution. Acidity is defined as a pH of less than 7.
Louis Missouri USdied January 4 1965 London England American-English poet playwright literary critic and editor a leader of the Modernist movement in poetry in such works as The Waste Land 1922 and Four Quartets 1943. Eliot in full Thomas Stearns Eliot born September 26 1888 St. Select two compounds above and this calculator will predict whether or not the reaction will occur in waterThis is simply based on the solubility chart of inorganic compounds.
This is case of strong acid titrated with strong base so we expect pH at equivalence point to be that of neutral solution - that is 700. A double replacement reaction will occur if a formation of a precipitate gas or water takes place. On the calculation of the degree of saturation of seawater with respect to calcium carbonate under in situ conditions.
If you do not have a water analysis you can use. In reality the answer will be slightly different. With molecular formula CH3OH has a molar heat capacity C p of 811 Jmol K.
This calculator converts automatically the pressure to bar with the following conversion factor. The best part is that only 4 of patients which is actually one patient experienced moderate degree of feeling unreality which can be equal to. The obtained osmotic pressure with formula 2 is in psi pounds per square inch.
PKa the negative logarithm of the acid dissociation constant molecular structures molar weights density and melting and boiling. To find pH for a given molarity you need to know how to work with logarithmic equations and a pH formula. The pH scale ranges from 0 to 14 under usual conditions and measures the acidity of an aqueous solution.
Geochemica et Cosmochemica Acta 341261-1291. A typical C-C single bond has a dissociation. New determination of carbonic acid dissociation constants in seawater as a function of temperature and.
Goyet C and A. This compound is a _____ alkyne. First sulfuric acid has pK a1 -3 very strong acid but second dissociation step has pK a2 20 so it is much weaker.
Eliot exercised a strong influence on Anglo-American culture from the 1920s. What is the general formula for alkenes. Crystalline structures are ionic molecular or atomic structures that form three-dimensional figures.
Here we will study the pH value formula and how pH value is calculated in detail. Heat Capacity - C - is a characteristic of an object - the amount of heat required to change its temperature by one degree.

Calculate The Degree Of Dissociation Of Hi At 450 C If The Equilibrium Constant For The Diss Youtube

Calculate The Percentage Degree Of Dissociation Of An Electrolyte Xy 2 Normal Molar M Youtube
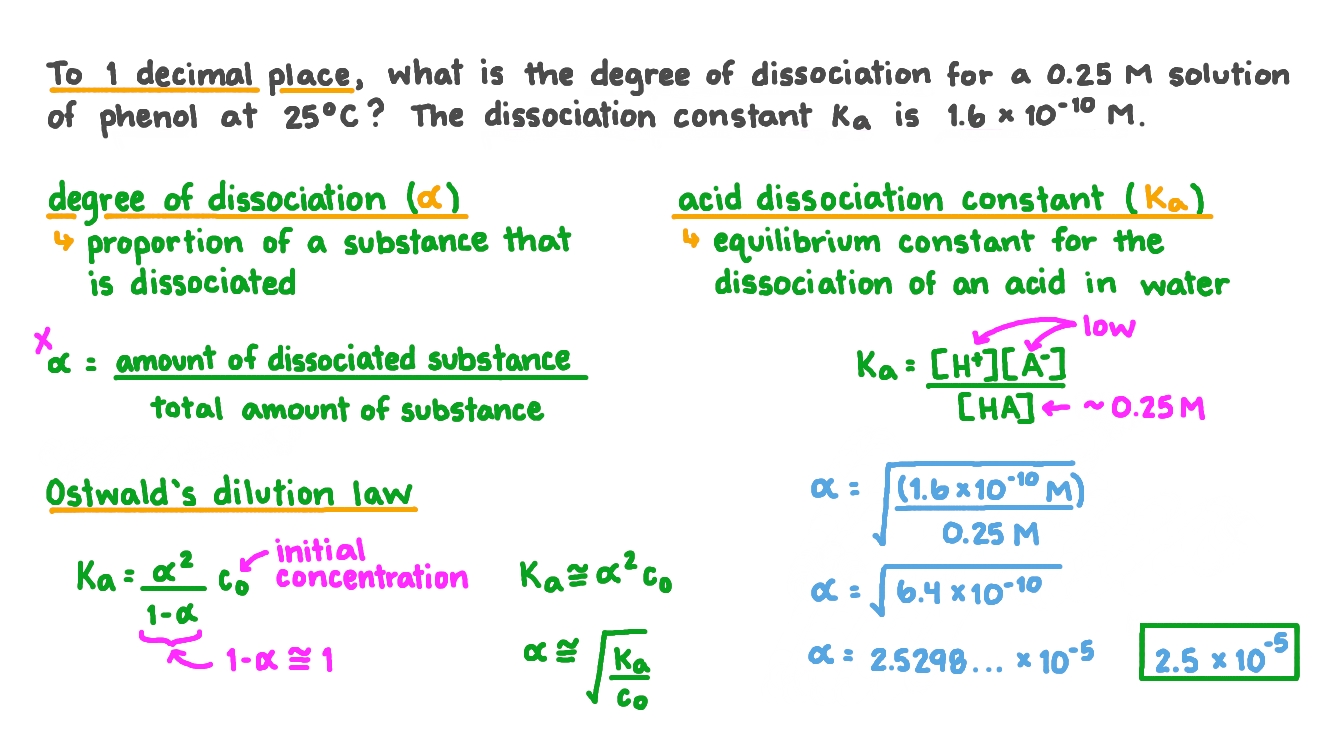
Question Video Calculating The Degree Of Dissociation Of A Solution Of Phenol Given The Acid Dissociation Constant Nagwa
No comments for "Degree of Dissociation Formula"
Post a Comment